How a car battery works
How a car battery works to make power
A car battery has positive and negative electrodes immersed in an electrolyte (a solution that supplies water and acid for an electrochemical reaction). The electrodes (plates) are made from dissimilar materials. When these two dissimilar materials are immersed in an electrolyte, they create a voltage potential.
The amount of voltage produced by each set of electrodes depends on the type of material used to build the electrode, but is typically 2.1 to 2.2 volts for a lead acid car battery.
In a typical car battery, the electrodes are built on a metal grid covered with a paste. For example, the positive electrode is commonly coated with lead dioxide (PbO2) and the negative electrode is coated with sponge lead (Pb). The electrolyte if sulfuric acid (H2SO4).
Creating electricity during the discharge cycle
Once you complete the electrical circuit and seek to draw power from the battery, the chemical reaction and electrical generation begin. The positive electrode (plate) is coated with lead dioxide (PbO2)—lead Pb and oxygen dioxide (O2). The PbO2 combines with the hydrogen H+ and sulfate (SO4) ions (from the sulfuric acid) to form lead sulfate (PbSO4) and water (H2O) on the surface of the positive plate.
Meanwhile, on the negative plate, Lead (Pb) from the negative active material reacts with the sulfate ions (SO42) from the sulfuric acid electrolyte to form lead sulfate (PbSO4) on the negative plate.
As you continue to discharge the battery, you’re basically using up the sulfuric acid and turning more of the electrolyte into water. That’s why a discharged battery freezes in winter—because the electrolyte has turned into water.
During the discharge process the coating on both plates is slowly being converted into lead sulfate (PbSO4), which is nonconductive, and the electrolyte (sulfuric acid) becomes weaker. That’s why the voltage drops; because there’s no longer a voltage potential between the two plates. Since the plates become less electrically conductive, internal conductivity drops. At a certain point, the battery can no longer produce electricity.
How fast does car battery discharge happen?
Let’s say you’re starting your engine and drawing 150-amps from the battery, but the engine isn’t in good shape. The starter motor cranks the engine, but either the fuel isn’t vaporizing properly of there’s an ignition issue preventing the engine from firing up.
If you continue to crank the engine at such a high load, you’ll discharge your battery at a much faster rate than if you were to leave the lights on for a long period. Here’s why; in order for the battery to produce electricity, the plate material with the sulfuric acid electrolyte must combine, and for that to happen, the acid must circulate into the pores of the plate coating and the water produced must also flow. Most of this reaction takes place on the surface of the plate. Once that voltage potential is used up, the acid would normally penetrate deeper into the plate coating. But on rapid discharge, the acid can’t penetrate into the coating that fast. So continued cranking quickly depletes your battery’s ability to create power.
How does a car battery recharge?
When you apply power to the battery, the chemical process reverses itself. The sulfate (PbSO4) in both plates splits into lead (Pb) and sulfate (SO4) and the water splits into hydrogen (H) and oxygen (O). The sulfate in the plates then combines with the hydrogen to form sulfuric acid (H2SO4). Meanwhile, the oxygen combines with the lead of the positive plate to form lead dioxide (PbO2).
As the battery reaches its “full” state, continued charging produces gassing —Hydrogen (H2) gas at the negative plate and oxygen (O2) at the positive. In effect, you’re driving off the water by continuing to charge the battery at a rate higher than in can accept. In older non-maintenance free batteries, the hydrogen and oxygen vented out the caps and was lost. That’s why you had to continually check the water level and add distilled water to bring it back to a proper electrolyte level. In a maintenance-free battery, the plate materials are slightly different and the venting is limited to reduce outgassing and the need for water replacement.
An automatic battery charger avoids this problem by tapering the charge level as the battery reaches full capacity.
Why car batteries die
Shedding
Car batteries don’t last forever, even when they’re properly maintained. Over time, the lead coating on the grids begins to soften and “shed.” Softening and shedding happens faster in a battery that’s abused through multiple deep discharge cycles caused by extended cranking or leaving your lights on or driving with a failing charging system that doesn’t recharge or overcharges the battery.
As the lead coating sheds, it falls to the bottom of the battery and accumulates in a sediment trap cavity 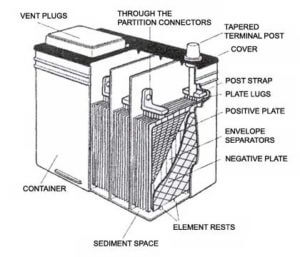 below the plates. The sediment trap is designed to accumulate this material without it contacting the plates. But, as the shedded fills the sediment trap, it can eventually reach the point where it comes in contact with the positive and negative plates,
below the plates. The sediment trap is designed to accumulate this material without it contacting the plates. But, as the shedded fills the sediment trap, it can eventually reach the point where it comes in contact with the positive and negative plates,
That causes a short between the contacted positive and negative plates. You’ll notice this kind of failure if you drive your car and it start and runs just fine, and the next time you go to start it, it’s completely dead.
According to a 2010 BCI Failure Mode Study (BCI=Battery Council International — the battery trade association), shorted batteries account for 18% of all battery failures.
Battery Sulfation
Car battery plate sulfation occurs when you leave a battery in a discharged or partially discharged state for long periods. How can a typical driver destroy a battery due to sulfation? Easy. Here’s an example. You start your car on a cold day. You then turn on the electric rear window defroster, crank up the heat and turn the blower to it’s highest setting. Then you turn on the headlights, wipers, and heated seats.
Here’s how much power you’re drawing:
Wipers 7.5-amps
Headlights 18-amps
Parking lights 8-amps
Brake lights 11-amps
Blower motor 18-amps
Rear window defogger 28-amps
Heated seats 5-amps (each)
Ignition system 8-amps
So, in addition to drawing at least 100-amps to start the engine, you’re also drawing slightly over 103-amps to run your accessories. Then you drive to the store or to work and you’re in stop and go traffic or your drive just a short distance. Your car is most likely equipped with at least a 130-amp alternator. But alternators can only output roughly 30% of their maximum value at idle speeds. So while you’re at a stoplight or crawling in traffic, you’re pulling more power out of your battery than you’re putting back into it. An alternator must run at close to 2,000 engine RPM to output its full charge. If you repeat this start/drain cycle every day for a week during cold weather, you can easily run your battery dry and cause the plates to sulfate.
In other words, sulfation occurs when your battery is discharged and stays in that condition for a while. The sulfation is really the formation of hard lead sulfate crystals that fill the pores of the paste coating on the plates. The lead sulfate crystals act as an insulator that prevents electrical generation. Once the sulfation occurs and is serious enough, it cannot be reversed.
How to avoid car battery sulfation
If you drive in the conditions described above, it really comes down to this: either drive the vehicle longer and at higher speeds, or connect a charger when you arrive home, OR prepare yourself to buy a new battery every one to two years. It’s really that simple.
Other ways to prolong the life of your car battery
Clean the battery posts and terminals
You can’t always see battery terminal/post corrosion and any amount of corrosion can dramatically affect the recharging rate of your battery. Worse yet, corrosion puts added stress on your car’s alternator, causing it to run hotter and fail earlier.
Protect your car battery against overheating
Most car owners think cold weather kills their batteries. Nope. Your battery may drop dead in cold weather, but it was actually damaged during summer. Keep in mind that a car battery is a chemical reaction and that kind of reaction happens faster as conditions heat up. Underhood temperatures accelerate battery deterioration. Heat is such a factor that many car makers install battery insulators right at the factory. You may think they’re designed to keep the battery warm in winter. Not true. They’re installed to keep your battery COOLER and protect it from underhood heat. If you replace the battery, may sure you move the battery insulator over to the new battery.
Secure your car battery to prevent vibration
The battery hold-down mechanism is there for a reason. It’s to prevent the battery from vibrating. You may think that the battery is heavy enough to not jiggle while driving. You’d be wrong. It does move if not secured. If you skip the battery hold-down, you’ll kill the battery. It’s that simple.
©, 2016 Rick Muscoplat
Posted on by Rick Muscoplat
